Organic compounds: they have at least one carbon atom and one or more other element (hydrogen, halogen, oxygen, sulfur, phosphorus...) Volatile, their boiling point is low (they evaporate easily). Among the most common VOCs are butane, ethanol, acetone Acetone, or propanone, is the organic compound with the formula₂CO. It is a colorless, volatile, flammable liquid and is the simplest and smallest ketone. Acetone is miscible with water and serves as an important solvent in its own right, typically for cleaning purposes in laboratories. About 6.7 … Benzene is an organic chemical compound with the chemical formula C₆H₆. The benzene molecule is composed of six carbon atoms joined in a ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. This medication is used as an antiperspirant to treat feet that sweat or smell excessively. It is also used as a drying agent during wart treatment..Acetone
Benzene
Formaldehyde
Full Answer
What are volatile organic compounds?
Volatile organic compounds (VOCs) are emitted as gases from certain solids or liquids. VOCs include a variety of chemicals, some of which may have short- and long-term adverse health effects.
What are the different types of VOCs?
Classifications of VOCs. While the demarcation line between the Very Volatile Organic Compound (VVOC), Volatile Organic Compound (VOC) and Semivolatile Organic Compound (SVOC) classifications (see table above) is somewhat arbitrary, it does show the wide range of volatility among organic compounds.
What does VOC stand for in chemistry?
Volatile organic compounds (VOC) means any compound of carbon, excluding carbon monoxide, carbon dioxide, carbonic acid, metallic carbides or carbonates and ammonium carbonate, which participates in atmospheric photochemical reactions, except those designated by EPA as having negligible photochemical reactivity.
What are VOCs in indoor air quality?
For indoor air quality, ALL organic chemical compounds whose compositions give them the potential to evaporate under normal atmospheric conditions are considered VOCs and should be considered in any assessment of indoor air quality impacts. An organic compound is any of a large class of chemical compounds whose molecules contain carbon.

Are terpenes a VOC?
Volatile organic compounds (VOCs) are a large group of various compounds including natural compounds as terpenes, alcohols, but also carbonyl compounds as ketones, aldehydes, ethers, aromatic hydrocarbons, and acids, which are the main pollutants present in indoor air [1,2].
What temp do terpenes degrade?
around 100°F.Some terpenes will begin to evaporate off at temperatures as low as 70°F, although most will begin to degrade at around 100°F. This has real effects not only for the taste and smell of the cannabis product, but also its effects.
What do terpenes do?
Terpenes are naturally occurring chemical compounds found in plants and some animals. They're responsible for the aromas, flavors, and even colors associated with various types of vegetation. In terms of cannabis, terpenes are what make certain strains smell or taste different from others.
What happens if you Decarboxylate too long?
What happens if you decarboxylate too long? Heating weed for too long, or at too high of a temperature, can burn off cannabinoids and terpenes, making your weed ineffective.
Are terpenes volatile?
Terpenes are volatile phytochemicals which contribute to the characteristic aroma and flavor of cannabis. Moreover, terpenes possess relevant pharmacological properties including analgesic (Guimarães et al. 2013), anti-inflammatory (Cho et al.
What terpene gets you highest?
There at least 100 identified terpenes in the cannabis plant, but there are five terpenes that can really enhance your high.Myrcene. Myrcene is the most common terpene. ... Pinene. Pinene is why some strains smell like pine trees, and it is what gives pine trees their natural scent. ... Linalool. ... Limonene. ... Caryophyllene.
What strain has the highest terpenes?
The latest batch of White Runtz (#WRPGA003) happens to be the most terp-forward yet: 4.49% total terpenes, with 1.7% limonene, 0.47% trans-caryophyllene, 0.43% linalool and 0.3% beta-myrcene. Beyond a funky aroma and rich flavour, terpenes also influence how your weed will hit you.
What happens if you smoke terpenes?
Inhaling pure, concentrated terpenes can cause damage and irritation to the lungs and may cause further damage once absorbed into the bloodstream. Some terpenes are directly toxic as well — such as benzene or methacrolein.
Does heat affect terpenes?
Terpenes begin to evaporate off the plant when temperatures are too humid or too hot. In fact, terpenes begin to degrade the moment cannabis is harvested, and heat accelerates this process. Too-hot temperatures can zap terpenes and diminish their flavor and purported effects.
At what temperature do cannabinoids destroyed?
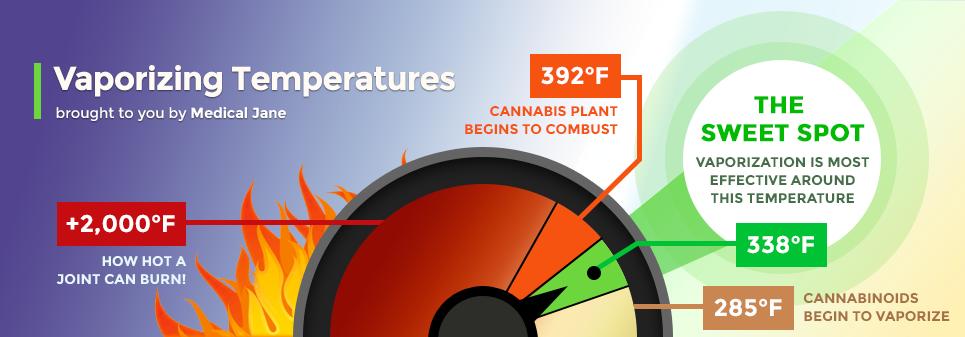
While “typical” baking temperatures may reach up to 450° Fahrenheit, THC will burn off completely at 390°, an easy temperature for any conventional oven or stove to achieve. However, THC begins to degrade much before the 390° mark.
At what temperature do trichomes melt?
At lower temperatures (290 to 330°F), your vaporizer will just begin to gently melt trichomes and release a vapor that may not be visible to the naked eye.
Does decarboxylation destroy terpenes?
Decarboxylation Destroys Terpenes For preparing CBD oil, this can be done using an oven or by heating dry herb in a hot water bath. Conventional oven drying methods will destroy almost all terpenes, and 50% of a bud's terpenes will be destroyed in just 5 minutes in a hot water bath.
What is a volatile organic compound?
Volatile organic compounds (VOC) means any compound of carbon, excluding carbon monoxide, carbon dioxide, carbonic acid, metallic carbides or carbonates and ammonium carbonate, which participates in atmospheric photochemical reactions, except those designated by EPA as having negligible photochemical reactivity 2.
What is a VOC in the air?
EPA formerly defined the regulated organic compounds in outdoor air as “Reactive Organic Gases“ (ROG). This terminology clarified its meaning as being limited to reactive chemicals. However, EPA later changed that terminology to “VOC”. Unfortunately, the use of the term “VOC” rather than ROG has created a misunderstanding when applied to indoor air quality. Many individuals and organizations, including manufacturers of building materials and products, and third party certification organizations have come to think of VOCs as “only those regulated by EPA for outdoor air”, and apply the same definition for indoor air purposes.
Why is EPA regulating VOCs?
While VOCs can also be a health concern outdoors, EPA regulates VOCs outdoors mainly because of their ability to create photochemical smog under certain conditions.
Why are organic compounds found everywhere?
Organic chemical compounds 1 are everywhere in both indoor and outdoor environments because they have become essential ingredients in many products and materials. Outdoors, VOCs are volatized or released into the air mostly during manufacture or use of everyday products and materials. Indoors, VOCs are mostly released into the air from the use ...
How are VOCs categorized?
VOCs are sometimes categorized by the ease they will be emitted. For example, the World Health Organization (WHO) categorizes indoor organic pollutants as: The higher the volatility (lower the boiling point), the more likely the compound will be emitted from a product or surface into the air.
What is the meaning of the word "volatility"?
Volatility is indicated by a substance's vapor pressure. It is a tendency of a substance to vaporize or the speed at which it vaporizes. Substances with higher vapor pressure will vaporize more readily at a given temperature than substances with lower vapor pressure.
Is volatility 4 higher or lower?
Since the volatility 4 of a compound is generally higher the lower its boiling point temperature, the volatility of organic compounds are sometimes defined and classified by their boiling points. For example, the European Union uses the boiling point, rather than its volatility in its definition of VOCs.
How to inhale cannabis extract?
The two primary methods for inhaling cannabis extracts are dabbing and vaping with cannabis e-cigarettes (CECs). 5,8 Dabbing is performed by placing a small amount of cannabis extract onto a heated surface while the user takes a large inhalation of up to an entire inspiratory capacity (<3 L). 5,8 CECs, commonly known as vape pens or oil pens, are compact e-cigarettes comprised of a single-use or refillable atomizer cartridge attached to variable or fixed-voltage batteries. The cartridge contains 0.3–1.0 g cannabis oil, a viscous substance that may contain up to 90% of the psychoactive Δ 9 -tetrahydrocannabinol (THC, mp = rt, 9 bp = 416 °C (ref. 10 )). 5 Dabbing and CEC use have quickly surged in popularity, and one recent study showed 19.5% of past-month cannabis users reported CEC vaping, and 14.6% reported dabbing. 11
Does terpene affect aerosolization?
Terpenes are shown to have a significant impact on aerosolization in both dabbing and CEC vaping. Curiously, opposite effects are observed for these two cannabis inhalation methods: higher levels of β-myrcene produces elevated levels of HPHCs during dabbing, but higher β-myrcene levels in SCO leads to lesser degradation and lower HPHC release for CEC vaping. For dabbing, this result is described using isotopic labelling, and it is shown that β-myrcene is more thermally labile than THC. The surface upon which aerosolization occurs is pre-heated to a desired temperature prior to administration of the material, and therefore all its components are subjected to the same temperature. Isotope labelling experiments indicate that β-myrcene has a 5–6 fold higher % yield of isoprene than THC. More facile routes to gaseous degradants, higher partitioning to the GP, and lower molar heat capacity are all factors that may explain the more extensive β-myrcene degradation compared to THC. Analogous findings consistent with this trend are likely for other terpenes with similar vapor pressures and molecular masses. Cannabis extracts used for dabbing typically contain cannabinoid acids, but these were not studied in this work given their lack of commercial availability for federally-funded academic research institutions in the United States of America as of this writing.
Methods
As part of an ongoing longitudinal study of the effects of e-cigarettes on adolescents, adolescent (aged 13–18 years) e-cigarette users (used an e-cigarette product on ≥1 day in the past 30 days and had at least 10 lifetime use episodes) were recruited from the San Francisco Bay area by using fliers and online advertising.
Results
Three hundred eighty-six adolescents were screened, 229 were found to be eligible, and 180 agreed to participate. After verbally reporting use within 24 hours, 29 participants admitted on their surveys to not using an e-cigarette product in the previous 24 hours and thus were excluded from analyses.
Discussion
To the best of our knowledge, this is the first study to report on the presence of VOC toxicants in adolescent e-cigarette users. Overall results reveal significantly greater toxicant exposure in adolescent e-cigarette users compared with their nonusing peers.
Conclusions
Although e-cigarette vapor may be less dangerous than combustible cigarettes, with lower overall exposure to VOC toxicants, with our findings, we challenge the idea that e-cigarette vapor is safe. Many of the VOCs we identified among e-cigarette users are carcinogenic, including propylene oxide, acrylamide, acrylonitrile, and crotonaldehyde.
Acknowledgments
We thank Mr Richard Ceballos III, Mrs Judy Gonzalez-Vargas, Dr Karma McKelvey, Mr Michael Berry, Mr Mark Thomas, and Mr Jerome Andres for their assistance with data collection and participant recruitment. We are also grateful to Lisa Yu and Peyton Jacob III for assistance with biomarker assays.
Competing Interests
POTENTIAL CONFLICT OF INTEREST: Dr Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies.
What is the most common solvent used in e-cigarettes?
In addition to these humectants, water is a common ingredient of e-liquids. PG and glycerol (commonly referred to as a “vegetable glycerin” in liquid formulations) are the most common vaporizing solvents used in e-cigarettes.
What are the substances in e-cigarettes?
Substances identified in e-cigarette liquids and aerosols include nicotine, solvent carriers (PG and glycerol), tobacco-specific nitrosamines (TSNAs), aldehydes, metals, volatile organic compounds (VOCs), phenolic compounds, polycyclic aromatic hydrocarbons (PAHs), flavorings, tobacco alkaloids, and drugs. Most reviewed studies have evaluated nicotine and impurities in the liquids such as TSNAs and nicotine-related impurities, while other studies have focused on identifying potentially harmful chemicals in the aerosol, such as carbonyl compounds, VOCs, TSNAs, metals, and silicates. Various chemical substances and ultrafine particles known to be toxic, carcinogenic, and/or to cause respiratory and cardiac disease have been identified in e-cigarette aerosols, cartridges, refill liquids, and environmental emissions. Some of the identified TSNAs, aldehydes, metals, VOCs, phenolic compounds, PAHs, and tobacco alkaloids are harmful or potentially harmful constituents, and their general health risks are described below.
What are the ingredients in e-cigarettes?
In general, e-cigarettes often contain ingredients such as propylene glycol (PG) and glycerol, mixed with concentrated flavors and, optionally, a variable percentage of nicotine.
What are the different types of humectants?
The study looked at several types of humectants, including dihydroxy (diols, glycols) and polyhydroxy alcohols. PG and glycerol were detected in all samples at concentrations ranging from 0.4 to 98 g/100 g (average 57 g/100 g) and from 0.3 to 95 g/100 g (average 37 g/100 g), respectively. Generally, lower levels of another solvent, ethylene glycol (average 10 g/100 g), were detected. 1,3-Propanediol was detected only in seven samples in the concentration range of 3.3–10 g/100 g. 1,3-Butanediol and diethylene glycol were negative in all samples. The presence of the major compounds glycerol and PG corresponded to the labeling in the majority of cases, except three products contained no labeling information at all. Glycerol was not labeled on five products despite being present. PG was not labeled in two products despite being present. In one case, “vegetal glycol” was labeled without specifying the exact chemical compound. Hutzler and colleagues (2014)analyzed 28 liquids purchased from 7 manufacturers in Germany and, like Hahn and colleagues, detected both PG and glycerol in all samples. Geiss and colleagues (2016)extrapolated lung concentration of PG and glycerol emitted from e-cigarettes using a smoking machine by measuring the average amounts condensed on the filter pad. The estimated lung concentrations were 160 and 220 mg/m3for PG and glycerol, respectively.
Is PG a solvent?
PG (also known as 1,2-dihydroxypropane, 1,2-propanediol, methyl glycol, and trimethyl glycol) is a clear, colorless, slightly syrupy liquid at room temperature. It is practically odorless and tasteless. It is used by the chemical, food, and pharmaceutical industries as a humectant to absorb extra water and maintain moisture in certain medicines, cosmetics, or food products. It is also used as a solvent for food colors and flavors, and in the paint and plastics industries. PG has been widely used for decades as a solvent for many intravenous drugs, and in some oral preparations such as cough syrups. PG was listed as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) in 1973 (HHS, 2015). Substances listed as GRAS are deemed as generally safe under conditions of intended use as a food additive. Thus, GRAS substances are safe for ingestion, but not necessarily for other routes of administration like inhalation. PG may exist in air in the aerosol form, but must be heated or briskly shaken to produce a mist. PG is also used to create artificial smoke or fog used in firefighter training and in theatrical productions.

Overview
General Definition and Classifications
- Volatile organic compounds (VOC) means any compound of carbon, excluding carbon monoxide, carbon dioxide, carbonic acid, metallic carbides or carbonates and ammonium carbonate, which participates in atmospheric photochemical reactions, except those designated by EPA as having negligible photochemical reactivity2. Volatile organic compounds, or VOCs...
Classifications of VOCs
- When discussing indoor environments, all organic chemical compounds that can volatize under normal indoor atmospheric conditions of temperature and pressure are VOCs. While the demarcation line between the Very Volatile Organic Compound (VVOC), Volatile Organic Compound (VOC) and Semivolatile Organic Compound (SVOC) classifications (see table above) …
Conclusion
- Reducing the concentration of VOCs indoors and outdoors is an important health and environmental goal. However, it is important to understand that there are VOCs of concern indoors and outdoors that do not impact photochemical oxidation and therefore are not regulated by EPA (42 U.S.C. §7401 et seq. (1970)). It is important to make and understand this distinction …
References